Climate change is a popular topic of discussion in today’s world. Human uses of fossil fuels are the main contributor to the rise in global temperatures. Despite this global problem, there are many potential solutions already, including the use of renewable energies and sustainable technologies. An often-overlooked renewable energy is hydrogen power.
Related Components | Test Your Knowledge 
This course will cover essential technical information regarding hydrogen power, specifically its applications, storage, and production. Additionally, it will discuss the use of hydrogen to power vehicles, comparing their advantages and disadvantages to electric vehicles (EVs).
2. Basic Concepts
What is Hydrogen Power?
Hydrogen power has many characteristics that make it an attractive alternative energy source. The first being that hydrogen is the most abundant chemical element in the universe. Here on Earth, it is found in water, which also happens to make up 71% of the planet’s surface. Hydrogen can also be converted into electricity at high efficiencies and can be stored in various forms such as a gas, a liquid, or a metal hydride. These various forms of storage also allow it to be transported easily. Lastly, hydrogen power has the potential to be a completely renewable fuel source.
Hydrogen is not only the most abundant chemical element, it also happens to be the lightest in mass. For instance, one gallon of gasoline weighs around 2.75kg, whereas one gallon of hydrogen weighs only 0.00075kg! This weight difference provides countless advantages for transportation applications. When transporting hydrogen, it needs to be in the form of either a liquid or compressed gas, the latter of which is the most common delivery method. Transportation often occurs through pipelines or through the use of super-insulated and cryogenic tanker trailers. Tanker trucks can hold a larger mass of liquid hydrogen compared to the transportation of gaseous hydrogen, making liquid hydrogen the preferred state for transportation.
What is a Hydrogen Fuel Cell?
In order to use hydrogen as a fuel source, a fuel cell is needed. A hydrogen fuel cell converts the hydrogen into electricity, creating water and heat as byproducts. This makes them ideal for many applications, from powering small electrical devices to powering large-scale manufacturing plants. Hydrogen fuel cells operate like batteries; however, unlike batteries, they do not need to be charged. They continue to produce electricity and heat as long as fuel (hydrogen) is supplied. They consist of an anode and cathode separated by an electrolyte material. When hydrogen is provided to the fuel cell a chemical reaction occurs, separating the electrons and protons that make up the hydrogen atoms. The electrons are sent to an external circuit creating a flow of electricity, while the protons combine with oxygen to create water as a byproduct.
Hydrogen fuel cells offer many advantages as an energy source that make them a great fit for future energy needs. They operate with a higher efficiency than internal combustion engines (ICEs) and produce zero emissions, unlike many other fuel sources. Hydrogen is also relatively easy to transport, as mentioned previously. Current research and development goals include reducing the cost of hydrogen as an energy source, improving its performance, and increasing the durability of hydrogen fuel cells.
3. Analysis
How to Produce Hydrogen Power
Hydrogen is rarely found in a pure form, but there are a few exceptions such as rock formations and other geological areas. Generally, hydrogen must be produced in large amounts in order to be used as a standard energy source. There are a few promising methods of producing hydrogen from water, including electrolysis, thermolysis, and photolysis.
Electrolysis is the process of using electricity to split water molecules into separate hydrogen and oxygen atoms. This is done using something known as an electrolyzer. Electrolyzers can vary in size, however, they all produce hydrogen through some sort of chemical process with water. They are constructed with an anode and cathode separated by an electrolyte material. Basically, they function in the opposite way of a fuel cell. Instead of using the hydrogen to create energy, energy is used to create hydrogen.
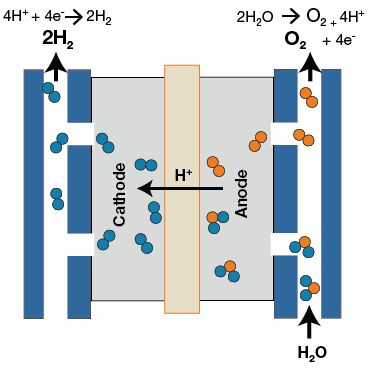
Figure 1. Electrolysis process using PEM Electrolyzer
Currently, there are a few different types of electrolyzers: polymer electrolyte membrane (PEM) electrolyzers, alkaline electrolyzer, and solid oxide electrolyzers. PEM electrolyzers operate by having water react at the anode to form oxygen and positively-charged hydrogen ions. These hydrogen ions then move to the cathode and combine with electrons to form hydrogen gas. In alkaline electrolyzers, hydrogen is generated on the cathode side and hydroxide ions move from the cathode, through the electrolyte, and to the anode side. Lastly, solid oxide electrolyzers use steam at the cathode side to form hydrogen gas and negatively-charged oxygen ions. The oxygen ions then move to the anode side to form oxygen gas and generate free electrons.
Electrolysis is important due to its ability to produce hydrogen with zero greenhouse gas emissions. The end-goal would be to utilize renewable energies to provide the electricity to be used for electrolysis. This can include solar, wind, geothermal, or nuclear power options. Using energy from the current power grid would not be a green solution, as the majority of the electricity generated today is done through various methods using fossil fuels that produce greenhouse gas emissions.
Another method of producing hydrogen is known as thermolysis. Thermolysis is a thermochemical process that uses energy to release hydrogen from the molecular structure of various resources. The most common method for thermolysis is through a process called natural gas reforming. It is a mature technology and process; in fact, over 90% of the hydrogen produced in the U.S. today is done through natural gas reforming.

Figure 2. The majority of hydrogen production today comes from natural gas reforming. Image source: www.energy.gov
Natural gas reforming is an endothermic process (heat is required) in which high-temperature steam, along with a fuel source such as methane, ethanol, or propane react to produce hydrogen. Generally, additional gases are produced as well. For example, in steam-methane reforming, hydrogen, carbon monoxide, and carbon dioxide all result from the chemical process. Undesirable impurities are removed such that a pure source of hydrogen results.
An alternative reforming method is known as partial oxidization. In partial oxidization, a limited amount of oxygen is used to produce a chemical reaction with one of the aforementioned fuel sources. The limited amount of oxygen allows oxidization to begin but not fully complete (hence the name). As a result, hydrogen and carbon monoxide are produced. Unlike natural gas reforming, partial oxidization is an exothermic process (produces heat). Additionally, it yields a smaller amount of net hydrogen per unit fuel when compared to natural gas reforming.
The last method of hydrogen production is photolysis. Photolysis describes the production of hydrogen using light is the primary source of energy. Various methods of photolysis exist, including photobiological processes, solar thermochemical processes, and photoelectrochemical processes.
- Photobiological – Photobiological processes of hydrogen production occur in microorganisms, such as microalgae or bacteria, using sunlight as the main energy source. With the introduction of sunlight, a process begins which breaks down organic matter and/or water into hydrogen. This method faces many challenges. It has a very low hydrogen production rate and low solar to hydrogen efficiency. However, it is still being considered as a long-term opportunity for environmentally friendly production of hydrogen.
- Solar thermochemical – The solar thermochemical process involves the “splitting” of water using a high temperature solar driven process. It has the potential to be a completely “green” process that utilizes sunlight and water. The process works using very high heat in the form of concentrated sunlight to drive chemical reactions that produce hydrogen. An additional material, such as copper chloride or cerium oxide, must also be included for the chemical reaction to occur. Many additional materials and chemical reactions are being studied to find the most optimal process. Some may require higher heat but have a simpler process, while others may have a more complex chemical process but a lower heat requirement.
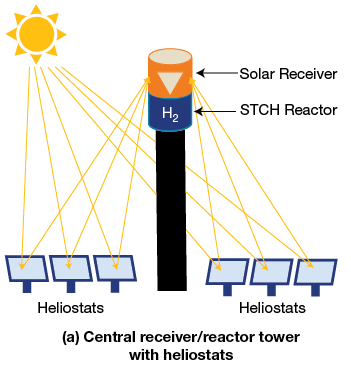
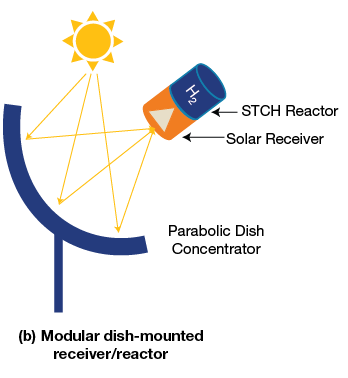
Figure 3. A visual representation of two different methods for concentrating sunlight. Image source: www.energy.gov
The last method of photolysis that can be used for hydrogen production is photoelectrochemical (PEC) water splitting. The PEC method is a longer-term option that is being explored; it uses a special semiconductor to split water into hydrogen and oxygen. The semiconductor material includes an electrolyte which, when energized with sunlight, performs a chemical reaction that splits water. In a way, it is similar to the technology used in photovoltaic cells. When sunlight hits a photovoltaic cell, a process occurs that converts the sunlight into electricity. Similarly, when sunlight hits a PEC semiconductor, a chemical process occurs that splits water into hydrogen and oxygen.
Thus far, we have covered various ways in which hydrogen can be produced. Currently, the most common forms of hydrogen creation are derived using natural gas as an energy source. Hydrogen produced in this manner is commonly referred to as blue or grey hydrogen, these names signify that greenhouse gases are still created during its formation. This is not ideal; however, it is still a step better than creating hydrogen using fossil fuels. On the other hand, hydrogen derived from fossil fuels is generally referred to as black or brown hydrogen.
Lastly, hydrogen produced entirely from renewable energy sources is called green hydrogen. For green hydrogen to become a viable fuel source, helping to reach climate change goals and net zero, the technology used to produce it must mature and production must increase. As production increases, prices will also come down, allowing hydrogen to become a reliable alternative energy source.
4. Applications
Hydrogen vs Electric Vehicles
While vehicles are not the only contributors to greenhouse gas emissions, it sure seems like they get the most attention when it comes to combating climate change. In recent years, electric vehicles (EVs) have become more prominent in news headlines as EV adoption has become more accepted among the general population. Governments are beginning to invest in nationwide charging networks, as well as offer incentives to encourage the public to purchase EVs. In addition, many legacy automakers have announced families of EVs for current and future production. However, with all the focus on EVs as the future of transportation, the lesser-known technology of hydrogen fuel cell electric vehicles (FCEV) offer another option for zero emissions transportation.
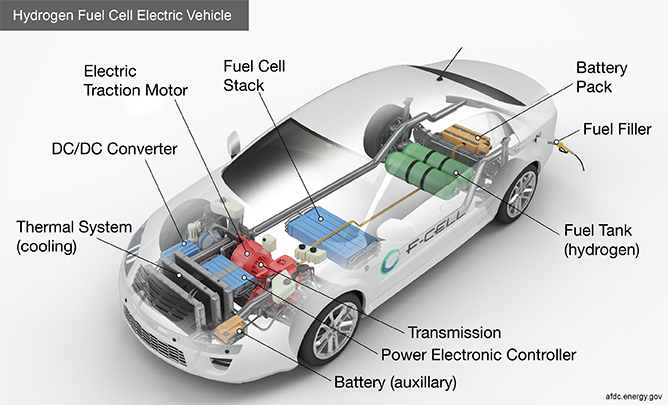
Figure 4. main components of a FCEV. Image source: afdc.energy.gov
Similar to EVs, FCEVs use electricity to power an electric motor or motors to propel the car. Unlike a traditional EV, the electricity used to power the motor comes from hydrogen rather than electricity stored in a battery. Hydrogen is stored in a fuel tank and is provided to a specialized fuel cell as needed. The fuel cell is where the hydrogen is used to generate electricity to drive the electric motor. Traditional batteries are still used on FCEVs for auxiliary power, as well as to provide supplemental power to drive the electric motor. The battery captures its energy through regenerative methods such as braking. The rest of the car consists of the same components as EVs, including DC/DC converters, a transmission, and thermal systems.
FCEVs offer some advantages over EVs, but they also have disadvantages. On average, fuel cell-powered vehicles are less efficient than electric vehicles. In fact, fuel cell vehicles are only around 38% efficient, whereas electric vehicles can be around 80% efficient. This means that somewhere around twice the amount of energy will be required by the FCEV to produce the same output power as an EV. Furthermore, refueling hydrogen vehicles is a very big issue. Due to the lack of hydrogen-powered cars on the market, it can be difficult to find a location where a hydrogen-powered vehicle can be refueled. If hydrogen is to compete in with electric vehicles, investment in fueling infrastructure is of paramount importance. On the other hand, EV adoption has given rise to many electric charging stations. In fact, the United States government has invested billions of dollars into funding a National Electric Vehicle Infrastructure program.
FCEVs have some key advantages over EVs. To begin with, refueling takes only a matter of minutes. Right on par with traditional gasoline powered cars, it takes three to four minutes to fill up a hydrogen tank. Furthermore, a full tank will offer a longer range than most current EVs. The expected range for a FCEV can be anywhere from 300 to over 400 miles. Electric cars can vary widely with their driving range, with less expensive cars ranging anywhere from 150 to 250 miles. However, it is worth noting the long-range edition of the Tesla Model S can achieve a range of up to 375 miles.
Both vehicle types produce zero emissions; however, the production and disposal of the materials need to be considered when discussing their carbon footprint. For instance, the production of lithium batteries is a major contributor to carbon emissions. In addition, there is also a large concern surrounding their disposal. It is estimated that by 2030, the number of batteries in need of disposal for vehicles will roughly equal the number being produced annually.
Overall, both FCEVs and EVs have their advantages, with EVs currently being the much wider accepted and adopted technology. Nevertheless, with future investment, acceptance, and cost reductions, hydrogen-powered vehicles could one day live side by side with electric vehicles on the roads of the future.
5. Current Applications of Hydrogen Power
Hydrogen power is already being used in many applications. These include business, personal, and industrial use cases. Companies are now outfitting warehouse vehicles such as forklifts and pallet jacks with hydrogen fuel cells. In addition, long-haul trucking companies like Toyota, Volvo, and Nikola are building and testing hydrogen powered semi-trucks. Furthermore, auto manufacturers such as Toyota, Honda, Hyundai, and BMW are also developing consumer level hydrogen vehicles, with some already available for sale. Buses for large city commuting are actively being tested to help cities become eco-friendly. Experimental projects for hydrogen-powered planes and trains are also being investigated.
Hydrogen-powered systems are beginning to make news headlines. In 2019, a boat by the name of the Energy Observer stopped in London, halfway through its multiyear journey to showcase the potential of renewable energies for future transportation. The Energy Observer generates energy through completely renewable methods. To begin, it is covered with 168 square meters of solar panels, capable of generating 28kW of power. Wind turbines are also included on board to help keep the 126kWh batteries charged. The battery energy is distributed for powering the motors and for onboard use by the crew. This might include power needed to run experiments, power monitoring and navigation equipment, or simply making coffee. Most notably though, a hydrogen fuel cell is integrated into the ship, which kicks in when the batteries drop below 60%. Hydrogen is stored in a 62 kg tank in the vessel. When needed, the hydrogen in the tank is replenished through a process using water directly from the sea. The water is first purified through a desalinator, then converted to hydrogen using an electrolyzer, and finally compressed and stored in the on-board tank. All these appointments come together to essentially make the Energy Observer a fully self-powered floating laboratory.
Another application where hydrogen power is already being used is backup power generation. Companies are beginning to look towards hydrogen to provide backup power when electricity is down. Examples include hospitals and data centers. In fact, Microsoft is working on replacing its diesel backup generators with hydrogen-powered battery storage in an effort to go carbon-neutral by 2030. Microsoft rarely uses its backup generators (which is a good thing); however that also makes it a great test case for the hydrogen solution. Microsoft’s goal is to provide customers with the five-nines of service availability, meaning that datacenters must be operational 99.999% of the time. Although the backup generators may barely be used, they are critical when consistent service is needed. Doing so using a zero-carbon emission, hydrogen-powered solution provides a great example of how hydrogen is beginning to replace fossil fuels in various applications.
6. Glossary
- Anode: the positively charged electrode where oxidation occurs in an electrochemical cell
- Cathode: the negatively charged electrode where reduction occurs in an electrochemical cell
- Cryogenic: extremely low temperatures below -150°C (-238°F) where gases and materials exhibit unique properties
- Electrolysis: the chemical process of using an electric current to drive a non-spontaneous chemical reaction
- Electrolyte: a substance that conducts electric current when dissolved in a solvent, often found in batteries and electrochemical cells
- Electrolyzer: a device that uses an electric current to induce electrolysis, separating compounds into their constituent elements
- Endothermic: a process or reaction that absorbs heat from its surroundings, causing a decrease in temperature
- Exothermic: a process or reaction that releases heat to its surroundings, causing a temperature increase
- Fuel cell: an electrochemical device that converts the chemical energy of a fuel, such as hydrogen, directly into electricity
- Photobiological: processes or phenomena that involve the interaction of light with living organisms or biological systems
- Photolysis: the chemical decomposition or splitting of molecules using light energy
- Thermolysis: the chemical decomposition or breakdown of a substance through the application of heat
*Trademark. element14 Community is a trademark of Avnet. Other logos, product and/or company names may be trademarks of their respective owners.

Test Your Knowledge
Power Skills 6

Complete our Essentials: Power 5 course and take the quiz to earn this badge.
Are you ready to demonstrate your wind power knowledge? Then take a quick 10-question multiple choice quiz to see how much you've learned from this module.
To earn the Essentials Power 6 Badge, read through the learning module and attain 100% in the quiz.