Solid-state batteries are getting closer to reality, but there are still challenges that need to be solved.
As modern devices become more and more ubiquitous, the technology to power them is pushed to the forefront. Traditional lithium-ion battery technology has almost exhausted its potential and is nearing energy density limits. Additionally, the use of liquid/polymer electrolytes in traditional Li-ion batteries can create safety hazards, such as leaking and in some cases, ignition. Solid-state batteries (SSB) address many of the limitations of the current technology, and with their rate of development, will soon become commonplace in electronic devices.
How Does a Battery Work?
Batteries consist of an anode (negative electrode) and a cathode (positive electrode), with an electrolyte between them. The discharging and charging of a battery is essentially a chemical reaction. As an example, in a lead-acid battery, the electrolyte is a mixture of sulfuric acid and water. When the battery is discharging, this chemical reaction occurs at the anode:
Pb + HSO4- → H+ + 2e-
While at the cathode:
PbO2 + 3H+ + HSO4- + 2e- → PbSO4 + 2H2O
Yielding overall:
Pb + PbO2 + 2H2SO4 → 2PbSO4 + 2H2O
As the battery discharges, electrons are released. The chemical reaction causes the sulfuric acid to break down into water, becoming more diluted. The charging process reverses this reaction, building back the acid molecules and storing the energy.
Lithium-ion batteries work in much the same way, transferring charged Lithium (Li+) ions between anode and cathode. There are several types of Li-ion batteries, including:
- Lithium Cobalt Oxide (LiCoO2 or LCO) — Batteries for devices such as cell phones, computers, and other peripherals
- Lithium Nickel Manganese Cobalt Oxide (LiNiMnCoO2 or NMC) — Commonly found in batteries for electric vehicles
- Lithium Nickel Cobalt Aluminum Oxide (LiNiCoAlO2 or NCA) – Also commonly used in batteries for EVs
- Lithium Manganese Oxide (LiMn2O4 or LMO) — Used in typical batteries for power tools
- Lithium Titanate (Li2TiO3 or LTO) — Also used in batteries for power tools, in addition to medical applications
- Lithium Iron Phosphate (LiFePO4 or LFP) — Also found in EV batteries, as well as batteries for industrial and power applications
Li-ion batteries have several advantages over batteries with traditional chemistries. With Li-ion, the electrodes do not dissolve in the electrolyte. As a result, Li-ion batteries deteriorate at a much slower rate and can survive many more charge/discharge cycles. Li-ion batteries are also more resistant to self-discharge. Additionally, lithium is very small and light, thus enabling the design of battery-operated devices to become increasingly smaller. However, Li-ion batteries have not yet replaced traditional lead-acid batteries in cars with internal combustion engines because they are more expensive.
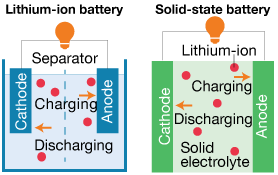
Figure 1. Traditional Li-ion battery compared to a solid-state battery
What is a Solid-State Battery?
Solid-state batteries use a solid electrolyte, as opposed to the liquid electrolyte found in traditional batteries. Solid-state Li-ion batteries work with a similar mechanism as traditional Li-ion batteries, and also contain an anode, cathode, and electrolyte. In a battery with a liquid electrolyte, a separator is installed between the anode and cathode (Figure 1). This separator is not needed in a solid-state design.
Development of solid-state batteries is ongoing, and in the next few years, batteries will be released that have several advantages over current technology. Solid-state batteries are expected to have a much faster charge rate, in addition to having a much higher capacity and output for the same footprint. Because the electrolyte is not in liquid form, solid-state batteries will be comparatively safer and satisfy all safety standards. Solid-state batteries are expected to be able to withstand and operate in a wider range of temperatures, and have a much longer lifespan when compared with traditional Li-ion batteries.
One promising characteristic of solid-state batteries is their resistance to heat. The faster the charging is, the more heat is generated. Because of their increased resistance to heat when compared to batteries with liquid electrolytes, solid-state batteries are expected to have much faster charge rates. A charge that would take 25 minutes for the current technology would take just 15 minutes for a solid-state battery. Faster charge rates, however, lead to degradation; a real phenomenon in traditional Li-ion batteries that solid-state batteries may be able to address. Solid-state batteries suffer less from the formation of dendrites, twig-like growths that cause the risk of short-circuit and capacity loss. Adden Energy has already achieved 5,000-10,000 charge cycles with a prototype of a coin-cell battery. In tests, QuantumScape’s solid-state EV battery has successfully undergone 400 cycles of 10 to 80% charging; the equivalent of covering 160,000 miles. Solid Power tested with periodic fast charges fast (every fifth cycle), a regimen similar to consumer behavior, and found 81% capacity retention after 650 charge cycles.
Types of Solid-State Batteries
There are two types of solid-state batteries, bulk and thin-film.
- Bulk batteries are larger and capable of storing a great deal of energy. They will be used in large applications such as EVs. Bulk solid-state batteries contain materials that are in powder or granular form.
- Thin-film batteries comprise a thin-film electrolyte on top of the electrodes. Because of their small size, they can not store a great deal of energy; however, they are well-suited for smaller devices, such as IoT devices and sensors.
Challenges in Development and Manufacturing
Solid-state Li-ion batteries have a their own set of problems to overcome. With traditional technology, the electrodes are submerged in the electrolyte, ensuring proper contact. With a solid electrolyte, maintaining contact can sometimes be difficult, especially when there is impact or slight deformation of the electrolyte or electrodes. There are also challenges in the manufacturing process. Many of the solid electrolytes are also very sensitive to moisture; even moisture in the air can cause degeneration.
Additionally, the interface connecting the solid electrolyte and positive electrode exhibits substantial electrical resistance. The ideal resistance value of the interface (unexposed to air) is 10 Ω-cm2 (Ohms per centimeter-squared). This escalates when the surface of the electrode is exposed to the atmosphere, degrading battery capacity and performance.
Exploring New Methods to Design Solid-State Batteries
Scientists from Tokyo Tech, AIST (National Institute of Advanced Industrial Science and Technology), and Yamagata University performed several experiments in an attempt to lower the resistance of the interface. Their results were promising; the fabricated batteries performed exceptionally well when compared to traditional Li-ion batteries.
The team used thin-film technology to create prototype batteries, each consisting of a lithium negative electrode, an LiCoO2 positive electrode, and a Li3PO4 solid electrolyte. The LiCoO2 layer was exposed to several elements, including air, nitrogen, carbon dioxide, hydrogen, and water. Surprisingly, water was the only molecule that drastically increased the interface resistance.
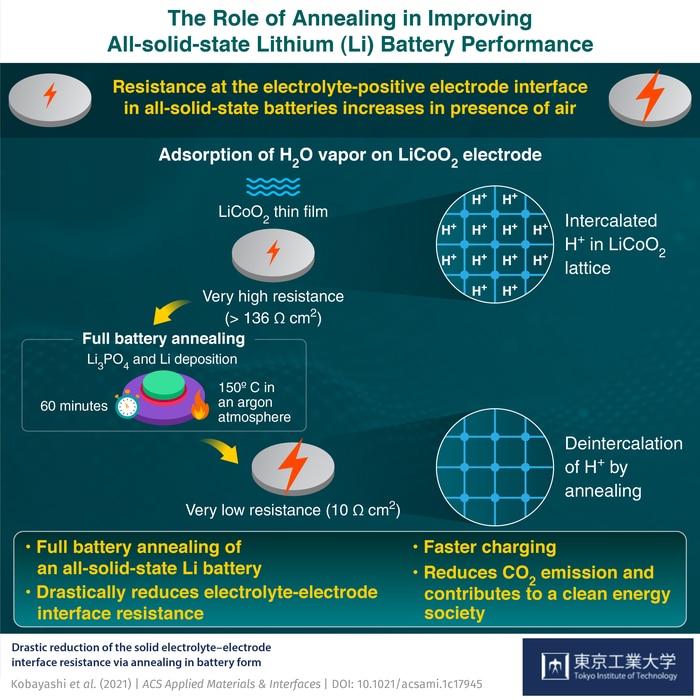
Figure 2. Findings from Tokyo Tech, AIST, and Yamagata Univ. study
Source: Shigeru Kobayashi and Taro Hitosugi of Tokyo Ins. of Tech.
After these tests, an “annealing” process was performed, where the sample endured an hour of heat treatment at 150°C in battery form. To the team’s surprise, the annealing process caused a reduction in resistance to 10.3 Ω-cm2, close to the original unexposed battery. The team hypothesized that the decrease in resistance was due to the removal of protons from the positive electrode (LiCoO2) structure during the annealing process.
Summing Up: Solid State Batteries
Solid-state battery technology is advancing quickly, and some time soon, solid-state battery technology may rival or exceed lithium-ion technology. Solid-state batteries eliminate many of the disadvantages of Li-ion batteries; however, for widespread adoption, their performance needs to match. Batteries require high energy density, fast charging, and long cycle life. Additionally, they need to be cost-effective. The research performed by the team from Tokyo Tech, AIST, and Yamagata University will pave the way.
How will solid-state batteries affect the design of current products, and what new products do you see them enabling?
Please tell us in the Comments section below.
