The evolution of battery technology in the last decades has allowed the development of many applications which simply wouldn't exist otherwise. Of course, one of the first things that comes to mind are electric cars. But what about battery powered vacuum cleaners (that are not just noisy but which actually clean)? Or new products like vapes, smart watches or e-bikes? They all require powerful yet lightweight batteries - and these batteries need to be developed, manufactured and tested. This is where Electrochemical Impedance Spectroscopy (EIS) can help.
EIS measures the impedance of a battery, which is its resistance to alternating current (AC) at different frequencies. Unlike direct current (DC) methods that only provide limited information, EIS captures a wide spectrum of internal reactions. By applying a small AC voltage and measuring the resulting current, EIS maps out the battery's impedance spectrum, revealing vital details about its internal chemistry and structure. One of the great advantages of using EIS for battery analysis is that it is completely non-destructive.
The data from EIS measurements are often represented in Nyquist plots (especially in Asia also known as Cole-Cole plot), which are graphs of the imaginary part of the impedance versus the real part. These plots can reveal a great deal about the internal processes and conditions of the battery, such as charge transfer resistance, mass transport limitations, and capacitive behavior.
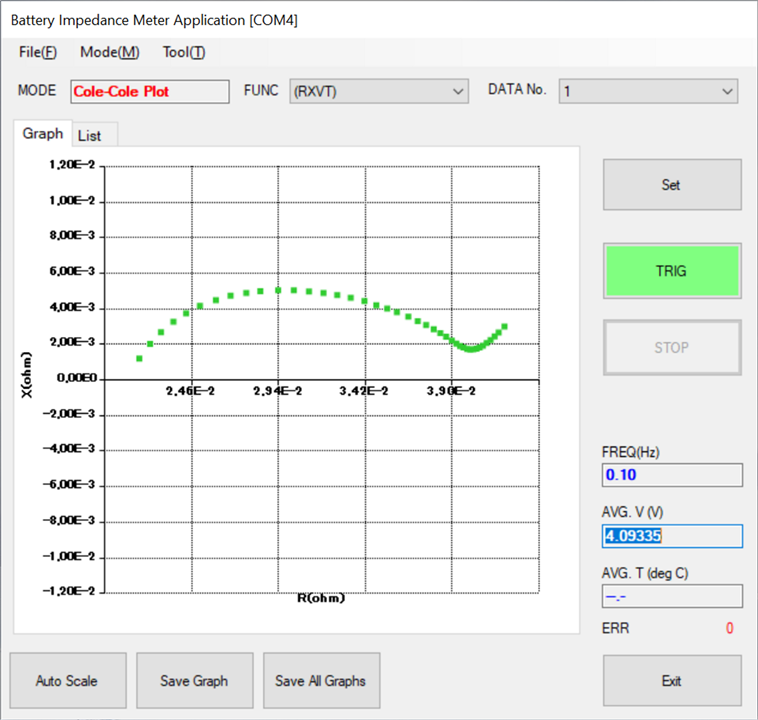
Let's take a more detailed look at what EIS allows us to analyze in batteries:
- Internal Resistance and Impedance: EIS is exceptionally effective in measuring the internal resistance and overall impedance of a battery. This includes both ohmic resistance (due to the electrolyte, separators, and other components) and charge transfer resistance (related to the electrochemical reactions at the electrodes).
- State of Charge (SoC) and State of Health (SoH): EIS can be used to determine the State of Charge, which is how much charge is currently stored in the battery compared to its maximum capacity. It also provides insights into the State of Health, which is an indicator of the battery's overall condition and how it has degraded over time.
- Electrode Material Properties: The technique helps in understanding the properties of electrode materials, such as porosity, surface area, and the nature of the electrochemical reactions occurring at the electrode surfaces. This is vital for battery design and innovation.
- Ion Transport Dynamics: EIS can analyze the kinetics of ion transport within the battery. This includes the movement of ions through the electrolyte and their intercalation (insertion) into the electrode materials, which are critical factors in battery performance.
- Diffusion Coefficients: The technique is useful in measuring diffusion coefficients of ions in the electrolyte and within the electrode materials. This information is important for understanding how quickly and efficiently ions can move, which impacts the charging and discharging rates of the battery.
- Electrolyte Conductivity and Composition: EIS provides insights into the conductivity of the electrolyte, which affects the internal resistance and overall efficiency of the battery. It can also detect changes in electrolyte composition that might occur during battery operation.
- Identification of Degradation Mechanisms: By analyzing changes in impedance spectra over time, EIS can identify specific degradation mechanisms, such as electrode material dissolution, electrolyte degradation, or the formation of resistive films on electrode surfaces.
- Temperature Effects: EIS can be used to study the effects of temperature on battery performance. Temperature variations can significantly impact the internal resistance and electrochemical reactions within the battery.
- Battery Design and Optimization: The detailed information provided by EIS helps in optimizing battery design, including electrode material selection, electrolyte composition, and cell construction.
- Quality Control and Testing: In a manufacturing context, EIS is a valuable tool for quality control, ensuring that batteries meet specific performance criteria and identifying defects or inconsistencies in production batches.
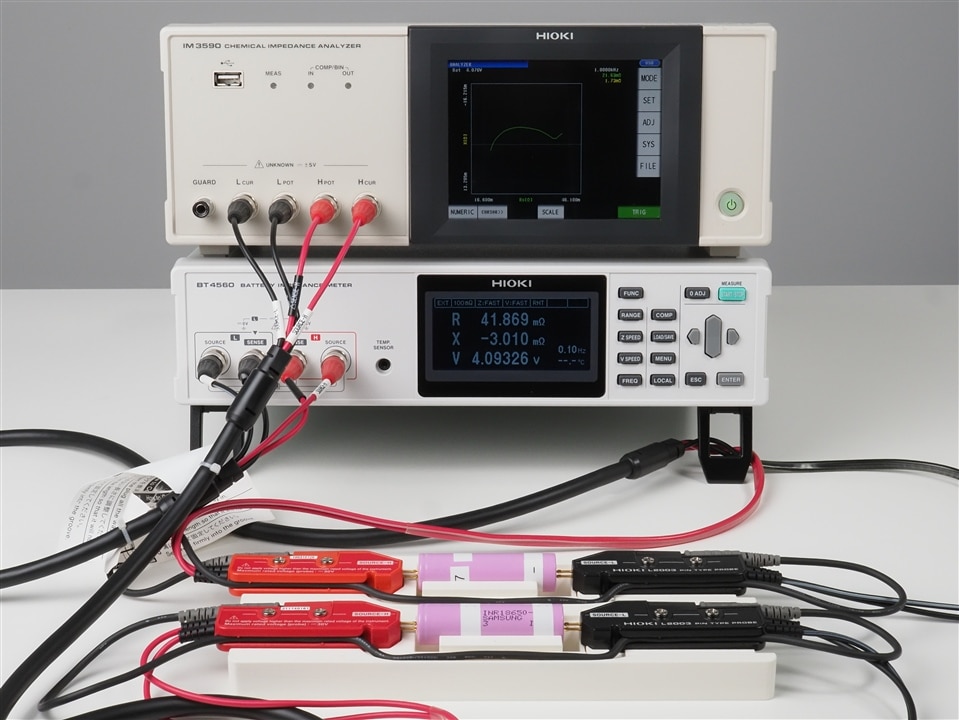
HIOKI has two instruments suitable for EIS in its portfolio. One is the IM3590 Chemical Impedance Analyzer and the second one is the BT4560 Battery Impedance Meter .
The IM3590 is perfectly suited for conducting EIS measurements in research and development of material science, chemistry, and electrochemistry. It's touch controlled and comes with a graphical interface that allows for Nyquist plots to be directly displayed on the screen. As it is typical for an impedance analyzer, frequency sweep points are set and stored directly in the instrument and no external control unit like a PC or PLC is required to make sweep measurements. That said, it is of course also possible to remote control the IM3590 - either through a PC and the included USB connector, or by adding an optional GPIB (Z3000 ), serial (Z3001 ) or LAN interface card (Z3002 ).
The frequency range of the IM3590 starts at 1mHz and goes up to 200kHz, making it suitable not only for the analysis of batteries but for a wide range of electrochemical studies and detailed impedance analysis across it's broad frequency range. Equivalent circuit analysis functions are natively included.

The BT4560 by itself is a battery impedance meter and it's perfect to test large capacity lithium ion cells thanks to it's "sweetspot frequency range" from 100mHz to 1050Hz and it's smallest resistance range of just 3mOhm. Being an impedance meter it doesn't natively support frequency sweeps, which is a requirement when performing EIS. That's why for EIS, the BT4560 needs to be controlled by a PLC or PC which sets the test frequencies and controls the sweep remotely. Equivalent circuit analysis can be performed with external tools like ZView.
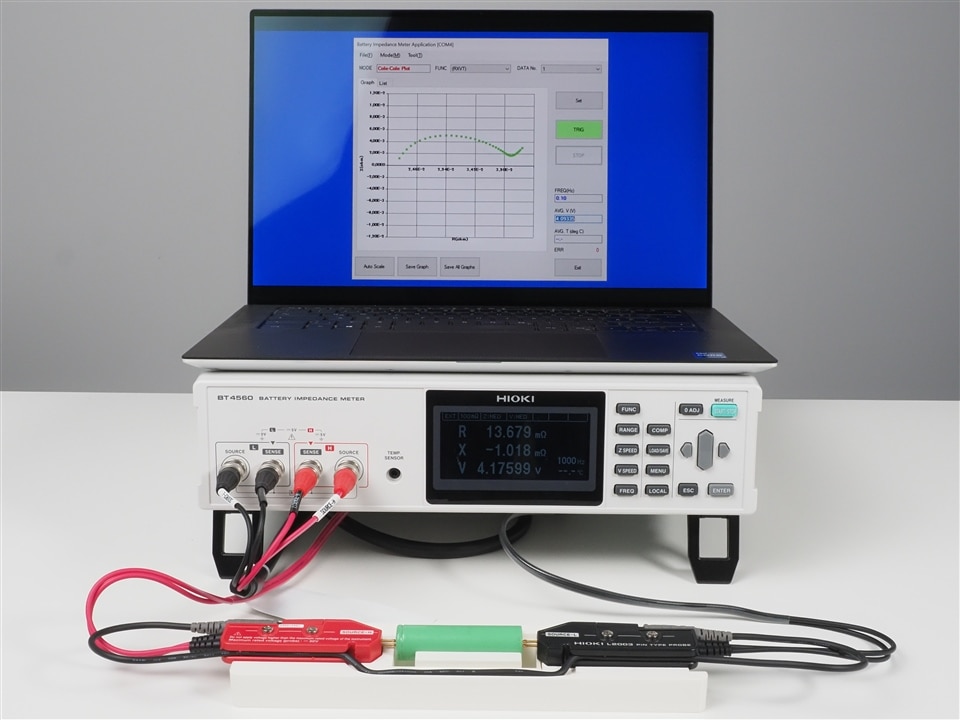
Comparing both instruments the IM3590 clearly looks like the superior instrument for EIS measurements. Then why would anyone consider the BT4560 as an alternative? Besides the price difference the smallest impedance range of the IM3590 is 100mOhm, which still makes it perfectly suited for measurements of the 18650 cells which are shown in the photos used to illustrate this text. However, if you intend to measure large capacity cells then the 3mOhm impedance range of the BT4560 offers a clear advantage. This is simply because the full scale error when measuring 1mOhm values in a 100mOhm range is likely to have a noticable effect.

The measurement principle of both instruments is a 4-terminal-pair configuration which is designed to further reduces the influence of measurement cables compared to the more common 4-wire configuration. Suitable test probes from HIOKI are the L2002 clip type lead and L2003 pin type lead. While the L2002 cable is best suited to connect pouch type cells with a flat tab-style battery contacts or laminated sheet batteries, the L2003 is the right choice for cylindrical or prismatic cells.

You might have noticed in the above images that the BT4560 features are temperature sensor input. Recording the ambient temperature together with the impedance values does can be important in production environments as temperature does have an effect on the impedance value of a battery. The suitable temperature sensor for the BT4560 is called Z2005 .

As a conclusion, EIS is a great tool to help you analyze batteries on cell level, and instruments like HIOKI's BT4560 or IM3590 allow you to perform EIS measurements quickly, easily and for all batteries, regardless of their type, size or capacity.

.jpg-1440x400x2.jpg?sv=2016-05-31&sr=b&sig=8empKL0IjcxI2iUt1GmXevO2VSGDHA%2FQ%2FlaU%2FK%2FdTy0%3D&se=2026-02-17T23%3A59%3A59Z&sp=r&_=U8/L3Ap8dQAcjCDuVNJb0Q==)