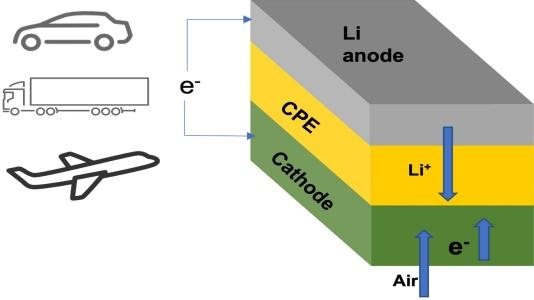
The new lithium-air battery cell consists of a lithium-metal anode, an air-based cathode, and a solid ceramic polymer electrolyte. (Image Credit: Argonne National Laboratory)
Electric car owners have longed for a battery that allows them to drive over a thousand miles on a single charge. Argonne National Laboratory and Illinois Institute of Technology developed a new lithium-air battery that could power cars, airplanes, and trucks in the future. The team claims this battery, which underwent testing for a thousand cycles, can store up to four times more energy compared to lithium-ion batteries.
The new battery has a solid electrolyte component rather than a liquid-based type commonly found in lithium-ion batteries. This helps prevent the battery from overheating and bursting into flames, which occurs in lithium-ion and other batteries.
"The lithium-air battery has the highest projected energy density of any battery technology being considered for the next generation of batteries beyond lithium-ion," says Argonne Distinguished Fellow Larry Curtiss.
Additionally, the battery chemistry could lead to increased energy density up to four times higher than lithium-ion batteries, resulting in a longer driving range. "For over a decade, scientists at Argonne and elsewhere have been working overtime to develop a lithium battery that makes use of the oxygen in air," said Curtiss." The lithium-air battery has the highest projected energy density of any battery technology being considered for the next generation of batteries beyond lithium-ion."
Previous lithium-air designs had the lithium in the metal anode passing through a liquid electrolyte to combine with oxygen during discharge, yielding lithium peroxide or superoxide at the cathode. While charging, the lithium peroxide or superoxide breaks down into lithium and oxygen components. This leads to on-demand energy storage and release.
The new solid electrolyte has a ceramic polymer material composition made of nanoparticle elements. This creates chemical reactions that yield lithium oxide during discharge. "The chemical reaction for lithium superoxide or peroxide only involves one or two electrons stored per oxygen molecule, whereas that for lithium oxide involves four electrons," said Argonne chemist Rachid Amine.
This is the first lithium-air design to achieve a four-electron reaction at room temperature. Air in the surrounding environment also provides oxygen while it functions. Such an implementation means the battery doesn't require any oxygen tanks.
The researchers used various techniques to determine that a four-electron reaction occurred. One crucial technique involved using transmission electron microscopy (TEM) on the cathode surface's discharged products. That TEM imagery showed insights into the four-electron discharge mechanism.
Other lithium-air test cells didn't have a long cycle life. The team concluded this drawback doesn't occur in the new battery design since its test cell lasts 1000 cycles, making it more stable during repeated charge and discharge.
"With further development, we expect our new design for the lithium-air battery to also reach a record energy density of 1200 watt-hours per kilogram," said Curtiss. "That is nearly four times better than lithium-ion batteries."
Have a story tip? Message me at: http://twitter.com/Cabe_Atwell
