The battery has a high-performance ABC discharge capacity. (Image Credit: Kenji Miyatake/Waseda University)
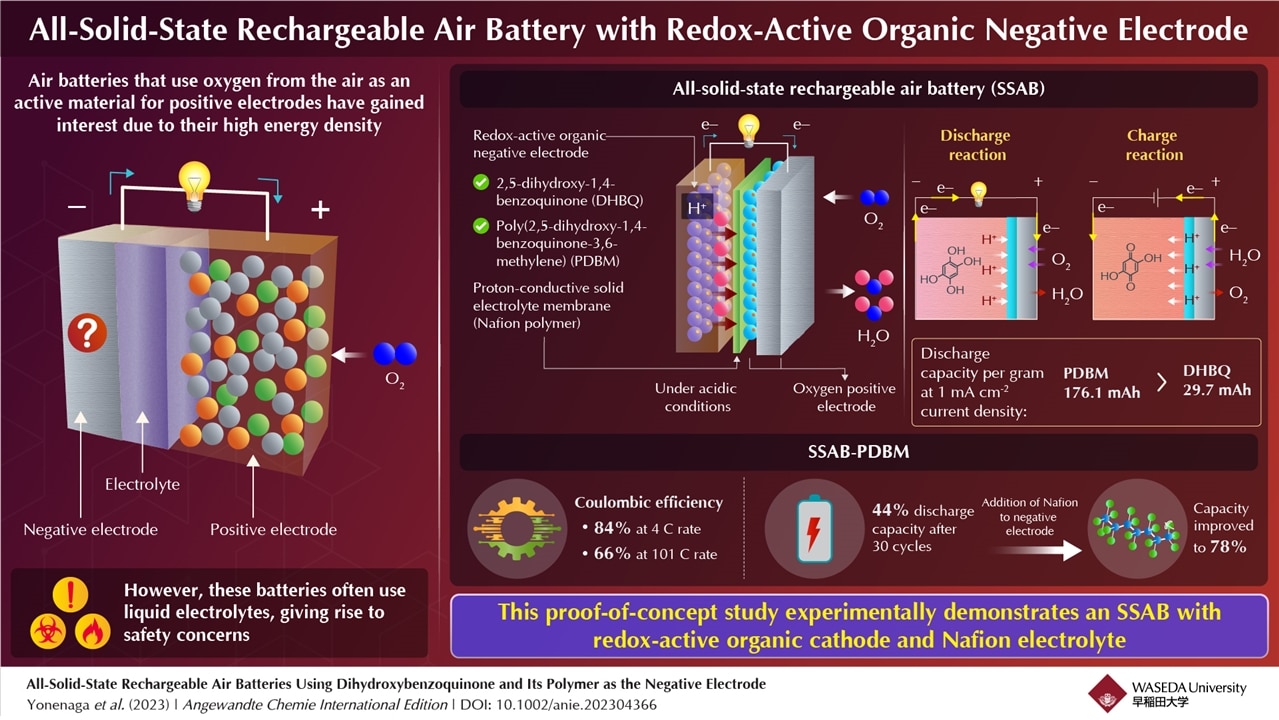
Traditional batteries use metal as negative electrodes, which leads to the formation of dendrites, structures that degrade battery performance. Redox-active organic molecules like quinone- and Anine-based molecules have recently been used as negative electrodes in rechargeable metal-air batteries with oxygen-reducing positive electrodes. In this case, protons and hydroxide are involved in the redox reactions. Japanese researchers created an all-solid-state rechargeable air battery (SSAB), observing its capacity and durability.
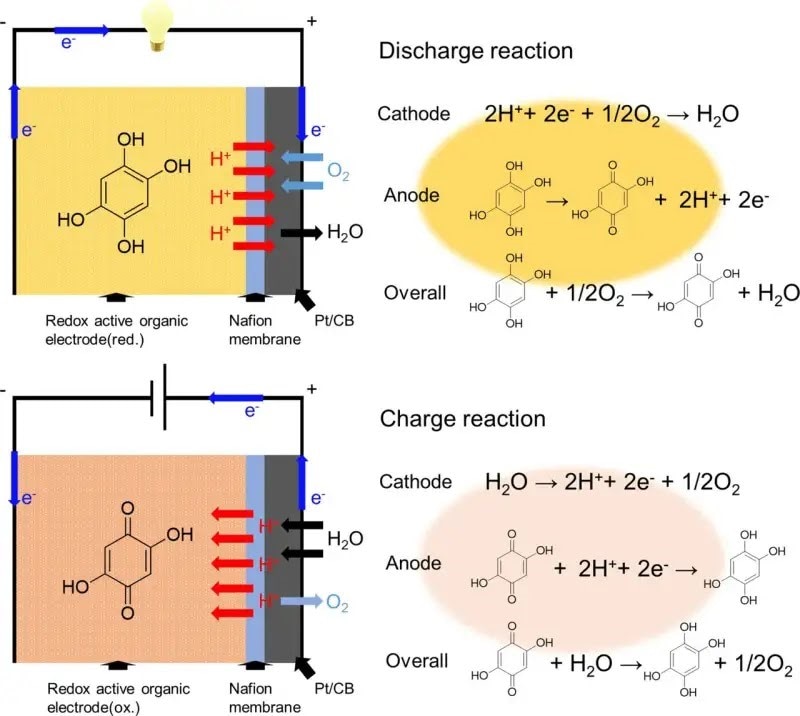
The battery's negative electrode consists of active materials called 2.5-dihydroxy-1.4-benzoquinone (DHBQ) and polymer poly(2.5-dihydroxy-1.4-benzoquinone-3.6-methylene) (PDBM). These offer stable and redox reactions in acidic environments. They also used Nafion (a proton-conductive polymer) for the solid electrolyte instead of standard liquid electrolytes.
"To the best of my knowledge, no air batteries based on organic electrodes and solid polymer electrolyte have been developed yet," says Miyatake.
Once they installed this battery, the team performed experiments to determine its rate characteristics, cyclability, and charge-discharge performance. They discovered that the SSAB didn't suffer from degradation if it contained oxygen and water. Also, replacing the redox-active molecule DHBQ with the polymeric counterpart PDBM formed an improved negative electrode. Although the SSAB-DHBQ had a 29.7 mAh gram-discharge capacity, the SSAB-PDBM was valued at 176.1 mAh.
The team's all-solid-state rechargeable air battery has a dihydroxy-benzoquinone-based organic negative electrode and Nafion polymer electrolyte. (Image Credit: Kenji Miyatake/Waseda University)
In addition, the team discovered the SSAB-PDBM's coulombic efficiency reached 84% at a 4°C rate that gradually decreased to 66% at a 101°C rate. Even though the SSAB-PDBM dropped to 44% after 30 cycles, the team improved it to 78% by boosting the negative electrode's proton-conductive polymer content. Electron microscopic images verified that adding Nafion boosted the durability and performance of the PDBM-based electrode.
The team is hopeful that their new battery will lead to more advancements. "This technology can extend the battery life of small electronic gadgets such as smartphones and eventually contribute to realizing a carbon-free society," said Miyatake.
Have a story tip? Message me at: http://twitter.com/Cabe_Atwell
