Schematic diagram representing the redox process where the electrode material undergoes oxidization and aluminate anions are deposited. (Image Credit: Birgit Esser/University of Freiburg)
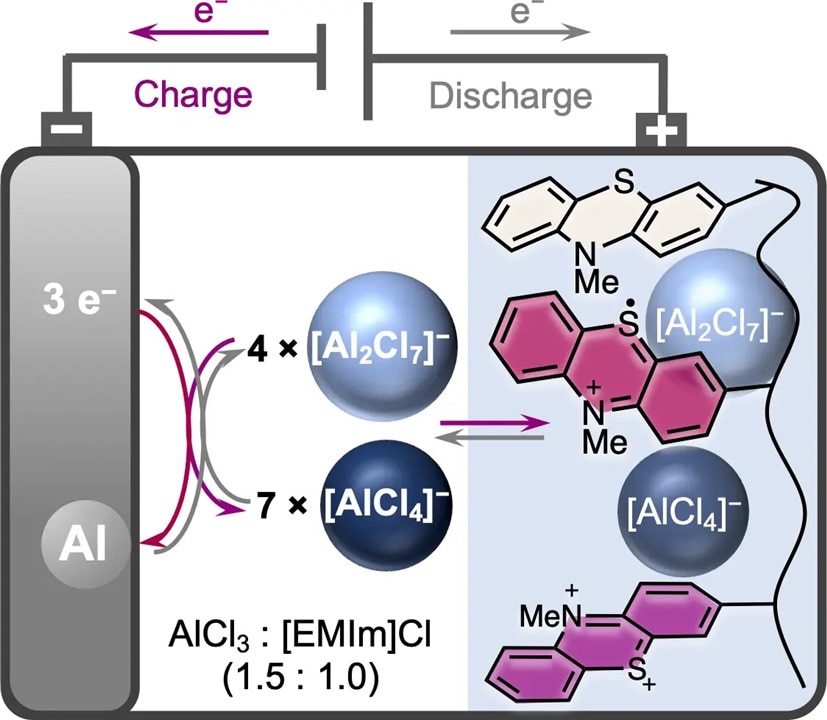
Aluminum-ion batteries could eventually replace lithium-based batteries. The transition makes a lot of sense because aluminum is highly recyclable, safe, and cost-effective, and there’s plenty of this material available. However, aluminum-ion battery technology is still in its infancy since researchers are searching for suitable electrode materials to achieve sufficient storage capacity. Scientists from the University of Ulm and the University of Freiburg created a positive electrode made of an organic redox polymer.
“The study of aluminum batteries is an exciting field of research with great potential for future energy storage systems,” says Gauthier Studer. “Our focus lies on developing new organic redox-active materials that exhibit high performance and reversible properties. By studying the redox properties of poly(3-vinyl-N-methylphenothiazine) in chloroaluminate-based ionic liquid, we have made a significant breakthrough by demonstrating for the first time a reversible two-electron redox process for a phenothiazine-based electrode material.”
According to the team, their polymer electrode material performed better than graphite during tests. It also stored 167 mAh/g of capacity and retained 88% capacity after 5,000 charge cycles at 10 C (charge/discharge rate of six minutes). If it takes longer to charge and discharge, the battery goes back to its original capacities. The team called this a huge step forward in their research.
The team says the electrode material oxidizes while the battery charges, which takes up complex aluminum anions. This allows the organic redox polymer poly(3-vinyl-N-methylphenothiazine) to reversibly add two [AlCl4]− anions at potentials of 0.81 and 1.65 volts while charging. An ionic liquid ethyl methylimidazolium-chloride serves as the electrolyte with the aluminum chloride.
“With its high discharge voltage and specific capacity, as well as its excellent capacity retention at fast C rates, the electrode material represents a major advance in the development of rechargeable aluminum batteries and thus of advanced and affordable energy storage solutions,” says Birgit Esser.
Have a story tip? Message me at: http://twitter.com/Cabe_Atwell
