Scientists successfully synthesized graphyne, which could lead to fast electronics. (Image Credit: Y Hu et al., Nat Synth, 2022)
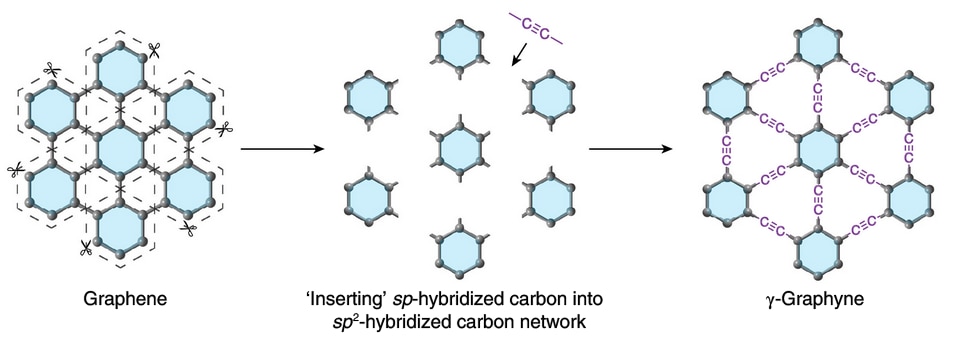
Graphene has been hailed as the "wonder material" ever since its 2009 synthesis, serving applications in electronics, medicine, energy, and various industries. Until now, graphyne, an identical material with minor differences that could lead to faster electronics, hadn't been synthesized. University of Colorado Boulder and Qingdao University of Science and Technology researchers recently synthesized graphyne in bulk. Graphyne has a layer of carbon atoms arranged in a symmetric lattice, making it similar to graphene. Additionally, graphyne's carbon atoms bind together in single, double, and triple bonds, making it different from graphene.
This study focuses on y-graphyne (gamma graphyne), graphyne's most stable isomer. Initially, synthesizing graphyne involved irreversible chemical reactions. This also meant that improper carbon atom arrangements could not be altered, destabilizing the lattice. So the team applied alkyne metathesis to rearrange the carbon chains' chemical bonds. That way, each molecule could switch its own part with another molecule. This approach relies on metal catalysts to rearrange benzene rings in a periodic lattice linked by triple bonds.
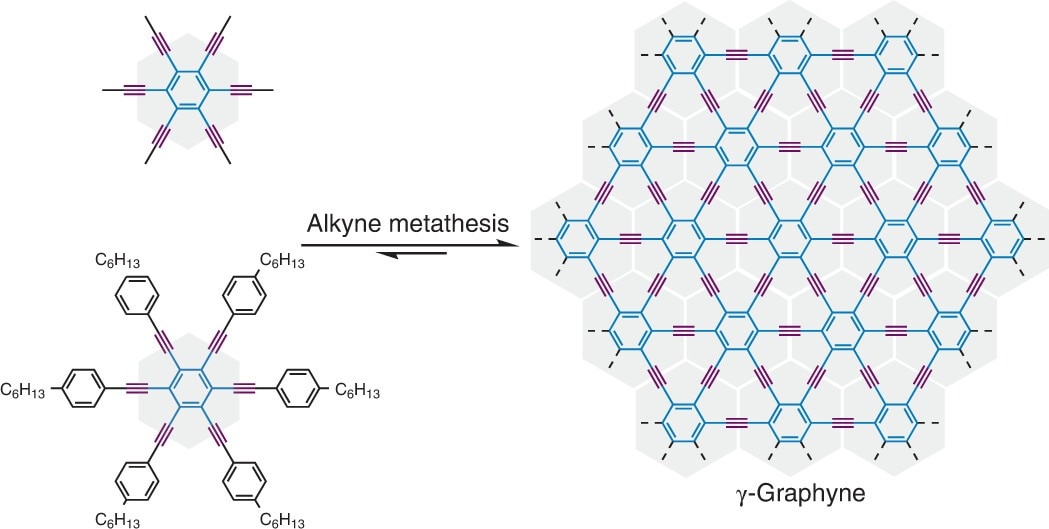
The team used alkyne metathesis to rearrange the chemical bonds in the carbon chains, allowing a molecule to switch out its own part with another. (Image Credit: Y Hu et al., Nat Synth, 2022)
Chemical reactions have kinetic control, meaning the ratio of the products is reliant on the rates at which it's formed. Then, there's also thermodynamic control, resulting in a more stable product. Producing graphyne meant the team needed to balance those reaction control techniques. This was achieved by applying two benzene derivatives to create the graphyne, and it took several days for a black solid to emerge from the y-graphyne solution.
Graphyne has potential applications in the semiconductor industry largely due to its mechanical, electronic, and optical properties. Its symmetry makes the electronic properties direction-dependent, differing from graphene. In addition, it doesn't need to undergo doping due to the conducting electrons. As a result, these make it much more applicable for semiconductors compared to graphene.
Have a story tip? Message me at: http://twitter.com/Cabe_Atwell
